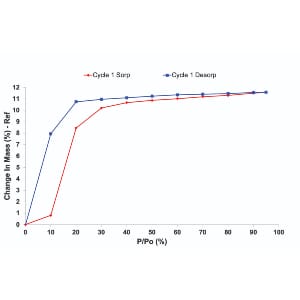
Water is commonly considered as the optimal dispersing medium for nanocellulose given its hydrophilic nature. However, studies have also proven otherwise that alternative organic solvents may give better dispersion quality. Herein, a thermodynamic approach is used to estimate the Hansen solubility parameters of bacterial cellulose (BC) and understand the dispersion of BC bundles in selected organic solvents (i.e. ethyl acetate, acetone and ethanol-water mixtures). The correlation between the BC bundle dispersion from different solvents and the aerosol particulate filtration performance of the resultant BC coated woven fabric is also presented. It is discovered that the best dispersion was achieved in a 60% ethanol-water mixture, resulting in enhanced aerosol particulate filtration performance. At a BC coating grammage of 0.5 g m-2, the filtration efficiency exceeded 80%, outperforming BC coating from water dispersion. These findings highlight the potential of understanding nanocellulose dispersion from a solubility parameter approach.
Joanne Lia, Yee Shuen Chama, Anett Kondorb, Eero Kontturic, Corinne Stoned, Mike Dennisd and Koon-Yang Leea,e*
a. Department of Aeronautics, Imperial College London, South Kensington campus, London, SW7 2AZ, United Kingdom
b. Surface Measurement Systems Ltd., London, HA0 4PE, United Kingdom
c. Department of Bioproducts and Biosystems, School of Chemical Engineering, Aalto University, FI-00076 Aalto, Finland
d. Defence Science and Technology Laboratory, Porton Down, Salisbury, Wiltshire, SP4 0JQ, United Kingdom
e. Institute for Molecular Science and Engineering (IMSE), Imperial College London, SW7 2AZ, United Kingdom