When CO₂ Meets Humidity:
What Happens When Amines Get Wet?
Most CO₂ capture research is done in dry lab conditions, but real systems rarely are. Even small amounts of moisture can change how CO₂ behaves in common amine liquids, sometimes improving performance and sometimes disrupting it.
This application note shows what really happens under realistic humidity. Some liquids absorb CO₂ more quickly with moisture present. Others become noticeably slower. In certain cases, humidity even reverses the expected results.
What you will learn:
– Unexpected reversals in performance
The amine you expect to win does not always win once humidity enters the picture.
– Humidity behaving in unpredictable ways
A small amount improves absorption. A little more creates diffusion barriers and stalls the process.
– Kinetics that defy the usual assumptions
One system becomes almost independent of CO₂ pressure. The reasons are not obvious until you see the data.
– Testing that reflects real conditions
Controlled CO₂ and humidity in DVS Carbon reveal behaviors missed by dry experiments.
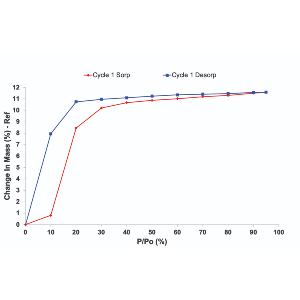
Download the application note to see full sorption isotherms, kinetic profiles, and mass-transfer analysis for MEA and MDEA (amines) under controlled humidity.