Sorption Overview Series #5
Comparing Sorption Methods
Comparing Sorption Methods
Lets talk measurement methods!
In this installment of our Sorption Overview series, we’re diving into the three most common methods used to measure gas or vapor uptake in materials: gravimetric, volumetric, and chromatographic (often referred to as breakthrough analysis).



Gravimetric Method
Gravimetric techniques determine gas or vapor uptake by directly measuring changes in sample weight using a highly sensitive microbalance. As conditions such as temperature and pressure or vapor concentration change, the balance detects shifts in net force, which correspond to the amount of sorbate absorbed or adsorbed by the material.
Because they rely on precise weight measurements, gravimetric methods such as Dynamic Vapor Sorption (DVS) are considered among the most accurate techniques for sorption analysis. They are especially useful for studying a wide range of materials under controlled and variable environmental conditions.
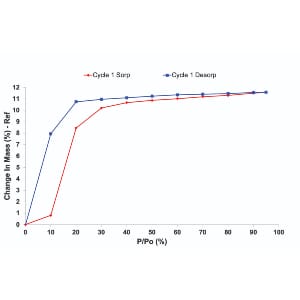
| Sample Mass Uptake
In a standard experiment, the sample is loaded in the balance and isolated in a chamber. The chamber temperature is carefully monitored, alongside the pressure/concentration of the gas phase. Change in mass given by the balance has three contributions: the change in sample mass given by uptake in the sample, a change in net buoyant force given by the change in density of the gas phase and a drag force of a gas flow on the sample pan. Buoyancy Force The buoyancy force is small for experiments at ambient pressure and temperature but can play a role at high pressure (>a few bar) where corrections may be required. Drag Force The drag force is only relevant when a dynamic mode is used, as the gas flow impinges on the balance parts and may need to be corrected through a blank. A way of minimizing both drag and buoyancy corrections is to construct the balance symmetrically, having a sample and a reference pan in identical configurations in the flow path. Experimental Design
Advantages of Gravimetric Methods One of the key advantages of gravimetric sorption methods is their flexibility in sample size. For single-component measurements, as little as 1–5 mg of material is often sufficient to yield highly reliable results. At the same time, gravimetric systems can accommodate much larger sample sizes, handling several grams or tens of cubic centimeters, depending on the material and application. The accuracy and range of measurements are directly tied to the performance and stability of the microbalance. For example, Surface Measurement Systems (SMS) offers advanced gravimetric analyzers with exceptionally high resolution:
This combination of precision and versatility makes gravimetric methods ideal for both delicate, small-scale experiments and larger, industrially relevant studies. Challenges and Considerations While gravimetric sorption techniques are known for their high sensitivity and accuracy, there are a few practical considerations to keep in mind. Gravimetric systems typically require a stable laboratory environment, particularly with respect to temperature and vibration control, to ensure consistent results. Additionally, extended equilibration times may be needed for certain materials or conditions, especially when studying slow diffusion processes. |

Volumetric Method
In volumetric methods, a cell containing the adsorbent material is dosed sequentially from a known volume of gas. The pressure is monitored as the probe sorbs into the material, and the equilibrium pressure is then used to calculate the final amount of gas remaining in the cell using an equation of state. The difference between the initial and final amounts of gas is equal to the amount adsorbed.
Sample Uptake
Advantages of Volumetric Methods
Challenges and Considerations Volumetric sorption techniques rely on indirect measurement via pressure, which introduces several important limitations:
|

Chromatographic / Breakthrough Methods
In chromatographic methods, the probe molecule flows through a packed bed or column containing the material to be characterized. Key variables including temperature, total pressure or concentration, and flow rate are continuously monitored at both the inlet and outlet of the column. These measurements allow for the calculation of a molar mass balance across the packed bed, providing insight into the sorption behavior of the material under dynamic flow conditions.
| Method Modes Chromatographic and breakthrough methods are dynamic flow techniques used to study how probe molecules interact with packed columns. Most systems operate in a carrier mode for easier pressure control. Probe introduction typically happens in two ways:
Experimental Process A sample-packed column is weighed before and after preparation, with thermal activation applied if needed. The system’s dead volume is measured using a non-interacting gas pulse. The probe pulse or front passes through the column, and sensors measure outlet concentration and flow to determine uptake via mass balance. Challenges and Considerations Controlling temperature, pressure, concentration, and flow precisely makes this method complex. It requires larger sample amounts (hundreds of mg to grams) and can be scaled for industrial simulation. Sample form affects performance, as fine powders may cause blockages. Additional Capabilities These methods uniquely provide multicomponent adsorption data and insight into adsorption kinetics critical for scaling processes. Surface Measurement Systems offers the iGC-SEA and BTA Frontier analyzers as leading tools for these analyses.
|
Check back soon for the conclusion of the series!
Don’t forget to check out the Sorption Hub user portal for more eLearning resources in materials characterization!