Capture of Hydrogen Sulphide and Sulphur Dioxide in MOFs
Watch now
Abstract:
Hydrogen sulphide (H2S) is a harmful chemical present in natural gas, biogas and emitted by different chemical industries, e.g., oil desulfurization process at oil refineries. H2S is considered as a major air pollutant due to its negative environmental impact, mainly associated with acid rain, and to high toxicity to humans leading to severe nervous system illnesses.
On the other hand, sulphur dioxide (SO2) considered as one of the most hazardous chemicals is a colourless, non-flammable gas with a strong odour. SO2 provokes severe health issues including alterations of the respiratory system (e.g., broncho-constriction in lung function). Typically, an exposure to only 1.5 ppm of SO2 for a few minutes can cause a temporary incapability to breath normally. Moreover, this chemical is highly soluble in water and forms sulphurous acid further converted to sulfuric acid, the main component of acid rain which can damage plants, accelerate the corrosion of metals and attack limestone, marble, mortar, etc. The harmful impact of this pollutant present in the atmosphere is also catastrophic in terms of global warming, ozone depletion and climate change.
Metal-Organic Frameworks (MOF) have been envisaged for the capture of H2S and SO2 however, some of them, with the main disadvantage of showing poor chemical stability. Thus, in this talk we present two MOF materials highly chemically-stable to H2S and SO2: MIL-53(Al)-TDC7 and MFM-300(Sc), respectively.
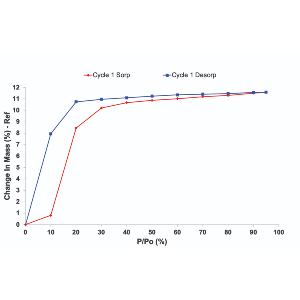
MIL-53(Al)-TDC is demonstrated to exhibit one of the highest H2S capture (18.5 mmol g-1 at 298 K and 1 bar) ever reported for a MOF, to the best of our knowledge, along with the retention of its crystalline structure after multiple H2S adsorption/desorption cycles and an excellent regeneration at relatively low temperature. MFM-300(Sc) is demonstrated to exhibit a SO2 uptake of 9.4 mmol g-1 at 298 K and 1 bar significantly higher compared to its Al- and In-analogues, along with the retention of this level of performance after multiple SO2 adsorption/desorption cycles owing to the high stability of its crystalline structure. Advanced experimental and computational tools have been further coupled to gain insight into the molecular mechanisms responsible for the adsorption of H2S and SO2.
Dr. Illich A. Ibarra
Associate Professor
Instituto de Investigaciones en Materiales, UNAM,