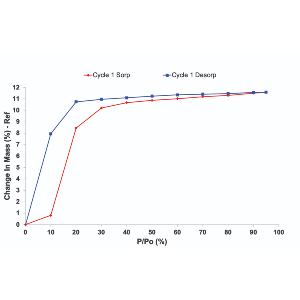
This workshop covers in-depth Dynamic Vapor Sorption (DVS) technology and its applications on studies of materials such as polymers, membranes, porous materials, thin films, and so on.
Polymers are widely used in various fields such as Energy and Environmental, Automotive and Aerospace, Marine and Biomedical amongst many other areas and DVS technique can provide insights into a variety of properties.
Agenda:
-Introduction to Surface Measurement Systems – Nachal Subramanian DVS
-Introduction and Basic Applications – Dr. Sabiyah Ahmed
-Vapour permeability in porous materials (particularly membranes and thin polymer films) – Meishan Guo
-Instrument and Software demonstration – Dr. Sabiyah Ahmed & Meishan Guo
This session will explore well-established vapor sorption techniques of Dynamic Vapor Sorption (DVS) and Inverse Gas Chromatography (IGC). With a focus on applications, the webinar delves into the physicochemical characterization capabilities offered by the DVS and IGC, specific to polymers and films.
In particular to IGC, practical examples and case studies are given that cover a wide range of relevant problems, including surface energetics, wetting behavior, composite adhesion/cohesion phenomena, solubility parameters, and glass transitions.
Related to DVS, examples, and case studies are reviewed that provide insight into moisture sorption properties, drying kinetics, vapor-induced phase changes, and vapor diffusion/permeability studies. Also, the hypenization of the DVS with video microscopy and Raman spectroscopy is discussed including examples on relevant materials.
Abstract:
Water should be considered an integral part of hair’s complex structure due to its considerable influence on fiber properties. However, this water content is not constant and varies with the relative humidity of the environment – and accordingly so do certain hair properties. These changing properties are behind the ability to create temporary so-called “water-set“ hairstyles – but they also represent the origin of eventual style failure. At the same time, consumers worry greatly about the water content of their hair and the potential for “drying out” – where there is a demand for “moisturizing” or “hydrating products” if this perceived situation is realized. The interaction of hair and water can represent a probe into the inner structure and there is a need for a means of accurately measuring hair’s water content.
Speakers:
Prof. Rupert Wimmer (BOKU)
Raphaela Hellmayr (BOKU)
Meishan Guo (SMS)
Dr. Sabiyah Ahmed (SMS)
Agenda:
– Introduction to Surface Measurement Systems (T. Schmid)
– Structure-related Vapor Sorption in Phenomena in Wood (R. Wimmer & R. Hellmayr)
– DVS Technique and Applications (M. Guo)
– iGC-SEA Technique and Applications (S. Ahmed)
– Live Q&A
Agenda:
-STFI Textile Essential
-Introduction to iGC
-Surface Energy and Heterogeneity -Sized and Unsized Fibres
-Effect of relative humidity
-Analysis of biomedical polymers
-Analysis of Cellulose fibres of different types
-Prediction of nanofiller-polyurethane composite interactions
-Q&A Discussion
Abstract:
Crystalline solids with controllable structures possess tailored porosity and large surface areas. This is particularly attractive for gas storage and separation applications. Physisorption of gases is a technique applied for the characterization of porous solids, such as zeolites and metal-organic frameworks (MOFs). Gravimetric vapor sorption and gas adsorption techniques measure promising functionalities such as removal of carbon dioxide (CO2) from the atmosphere or stability of sorbents in humid environments. Currently, both techniques are routinely applied for the characterization of porous solids to explore adsorption capacities and porosity. However, only a few studies focus on the pre-experimental conditions for the determination of gas/vapor sorption isotherms. Details of outgassing conditions, despite their importance, are often lacking in research publications. Outgassing at low temperatures of thermally stable material provides an incomplete cleaning of the porous surface. As a result, the ability of sorbents to store CO2 or water molecules is underestimated based on adsorption data. Contrary, outgassing of temperature-sensitive sorbent at elevated temperatures can cause irreversible structural changes which will have profound effects on the adsorption capacities. The impact of water adsorption on the structure was isolated by introducing partial pressures of water under a vacuum. Such measurements provided a true water adsorption isotherm without unnecessary interference from a carrier gas. CO2 adsorption data were measured from low pressures up to 1bar. CO2 adsorption in the presence of water on the sorbent was collected in a system under a vacuum. The results show that the performance of the sorbent can be significantly modified depending on outgassing conditions.
Oil pollution, from anthropogenic exploration activities, constitutes a serious risk to water resources globally. Different conventional sorbents have been used to remove oily contaminants from affected water bodies with varying degrees of success. In recent years, natural sorbents such as kapok fibres, rice husks, and chicken feathers have been explored as a substitute for conventional sorbents because they are cheaper, abundantly available and provide a means to develop a circular economy. The oil sorption capacity of these fibres is determined by their surface chemistry and microstructure–surface properties.
In this study, Inverse Gas Chromatography (IGC-SEA) was utilized to evaluate the surface properties of chicken feather mat – a natural sorbent and polypropylene pad – a synthetic organic sorbent. The BET, dispersive/specific (acid-base) surface energies, specific free energies of adsorption, and thermodynamic work of cohesion and adhesion for chicken feather mat and polypropylene pad were measured. The surface profiles demonstrated that both sorbents were energetically heterogeneous. However, the chicken feather mat surface energy profiles showed comparable values to the conventional polypropylene pad, widely used as an oil sorbent.
The vapor pressure of material is an important physical property that defines the amount of vapor molecules generated at its surfaces. Materials have tendency to enter the vapor phase by sublimation (solid-gas) or evaporation (liquid–gas). The vapor pressure of material at the thermodynamic equilibrium is only a function of temperature.
The knowledge of vapor pressure is highly desirable for liquids, oils, pesticides, fertilizers, and various substances in order to avoid the atmospheric accumulation of toxic compounds with low vapor pressure. The US Environmental Protection Agency (EPA) and the European Chemicals Agency (ECH) mandate the registration of vapor pressure of certain materials, because of the impact on the environment.
Furthermore, the vapor pressure can play a vital role in manufacturing, for example, organic solar films, where the progressive reduction of additives like plasticizers and UV absorbers as a result of evaporation from the surface can cause an unwanted decrease in their efficiency.
In this webinar, Knudsen Effusion Method and Static Method for determining the vapor pressure of materials will be discussed.
Wavy nickel nanowires (NiNWs) were immobilized on mesoporous silica (SiO2) aerogels by the sol−gel method. The catalytic activity of pure NiNWs and NiNW−SiO2 aerogel composites toward the CO2 hydration reaction (CHR) when they are in water was measured. Dynamic Vapor Sorption (DVS Vacuum) analysis was performed at levels of 50% CO2 and 50% H2O vapor for SiO2 aerogels, immobilized nickel nanoparticles (NiNPs) on silica aerogel and NiNW−SiO2 aerogel composites, in order to determine catalytic activity for CHR in the gaseous phase. The results from DVS Vacuum analysis (gaseous phase) and CHR (aqueous phase) showed that NiNW−SiO2 aerogel composites are good heterogeneous catalysts for CHR in both gaseous and aqueous phases but they are less active than NiNP−SiO2 aerogel composites.
Moisture and organic solvent sorption have a large impact on the mechanical, physical, and chemical properties of many materials.
This webinar will highlight a series of water and organic solvent sorption characterization methods for determining the hydrate and solvate formation of different materials. During this presentation, we are going to highlight a series of examples of hydrates and solvation formation and stability. Moreover, we are going to present a case study of solvate formation performed with DVS and in situ Raman spectroscopy.
Using a novel dynamic flow configuration, this gravimetric experimental system can measure both competitive multicomponent adsorption as well as water sorption and glass transition processes. This characterization technique can not only be used across a wide range of materials at different temperatures but is well suited for adsorption studies using organic vapors at high partial pressures.