Characterizing construction and building materials? Watch this free online workshop to discover how you can maximize the accuracy and detail of your research using Vapor Sorption Techniques.
Organized in partnership with the University of Applied Sciences in Wismar, this session is a vital resource for any working in the following fields of research:
-Wood
-Cement
-Building Materials
In the real world, capture/adsorption of CO2 is often a competition with other gas-phase species, most commonly water vapor. In some cases, the concentration of H2O vapor is much higher than that of CO2 further complicating the adsorption process.
This presentation, delivered by one of the world’s leading authorities in sorption science, Dr. Daryl Williams, will compare the use of two of the most commonly used experimental approaches for studying CO2 sorption in the presence of H2O vapor; gravimetric analysis using Dynamic Vapour Sorption (DVS) and breakthrough analyzer studies. To give the audience maximum insight, experimental details and case study comparisons of adsorbent performance will also be presented.
Organized in partnership with University of Namur.
Abstract:
Hydrogen sulphide (H2S) is a harmful chemical present in natural gas, biogas and emitted by different chemical industries, e.g., oil desulfurization process at oil refineries. H2S is considered as a major air pollutant due to its negative environmental impact, mainly associated with acid rain, and to high toxicity to humans leading to severe nervous system illnesses.
On the other hand, sulphur dioxide (SO2) considered as one of the most hazardous chemicals is a colourless, non-flammable gas with a strong odour. SO2 provokes severe health issues including alterations of the respiratory system (e.g., broncho-constriction in lung function). Typically, an exposure to only 1.5 ppm of SO2 for a few minutes can cause a temporary incapability to breath normally. Moreover, this chemical is highly soluble in water and forms sulphurous acid further converted to sulfuric acid, the main component of acid rain which can damage plants, accelerate the corrosion of metals and attack limestone, marble, mortar, etc. The harmful impact of this pollutant present in the atmosphere is also catastrophic in terms of global warming, ozone depletion and climate change.
Metal-Organic Frameworks (MOF) have been envisaged for the capture of H2S and SO2 however, some of them, with the main disadvantage of showing poor chemical stability. Thus, in this talk we present two MOF materials highly chemically-stable to H2S and SO2: MIL-53(Al)-TDC7 and MFM-300(Sc), respectively.
MIL-53(Al)-TDC is demonstrated to exhibit one of the highest H2S capture (18.5 mmol g-1 at 298 K and 1 bar) ever reported for a MOF, to the best of our knowledge, along with the retention of its crystalline structure after multiple H2S adsorption/desorption cycles and an excellent regeneration at relatively low temperature. MFM-300(Sc) is demonstrated to exhibit a SO2 uptake of 9.4 mmol g-1 at 298 K and 1 bar significantly higher compared to its Al- and In-analogues, along with the retention of this level of performance after multiple SO2 adsorption/desorption cycles owing to the high stability of its crystalline structure. Advanced experimental and computational tools have been further coupled to gain insight into the molecular mechanisms responsible for the adsorption of H2S and SO2.
This session will cover Metal Organic Frameworks (MOFs) that can be used in a range of adsorption applications, such as gas storage, carbon capture, separations, catalytic transformation, and drug delivery. In addition, they can be also used for thermochemical energy storage applications, whereby heating and cooling technologies use thermo-adsorptive effects.
The combination of hierarchical pore structure control of these materials and the selection of appropriate adsorbent enables entry and adsorption of small molecules on internal surfaces. Such processes are typically controlled by physisorption mechanisms governed by molecular size, polarity, and chemical nature of the sorbent surfaces. In some cases, specific chemical interactions can give rise to more strongly bound chemisorbed species which are in essence part in the design of heterogeneous catalysts
Water sorption, Organic sorption and Gas adsorption measurements on MOFs can provide valuable information about the effect of probe molecules on materials stability, kinetics, adsorption capacity, and energy requirements for the regeneration of adsorbents.
This webinar highlights an innovative experimental method for determining gas and vapor adsorption and co-adsorption isotherms on MOFs using a novel dynamic vacuum flow configuration in a broad temperature range.
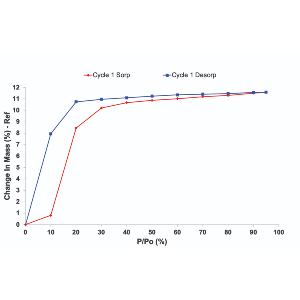
The interaction of a solid with its surroundings is through the available surface area for adsorption of gas or vapour molecules. This also allows probing of materials surface including irregularities and pores. One of the most successful methods is based on the BET method for gas adsorption onto a solid surface. The adsorption method of Brunauer, Emmett and Teller (BET) is based on the physical adsorption of a vapour or gas onto the surface of a solid. Traditionally, sorption studies were carried out at low temperatures to obtain nitrogen isotherms at 77 K, which were then used to calculate BET surface areas. Considering that material behavior varies with temperature, measurements at ambient temperatures may be more relevant and also allow the use of various gases and vapours.
The present study is devoted to a critical investigation of the specific surface area analysis of cellulosic materials, such as freeze-dried bacterial cellulose, cellulose nano-paper and crystalline cellulose powder by gas and vapour adsorption using dynamic vapour sorption (DVS) technique, volumetric technique and inverse Gas Chromatographic (iGC) technique and the favourable case of cellulosic materials is emphasized. Inorganic materials including the specific surface area standard materials can be successfully measured with these techniques, however, the applicability of these techniques on organic materials has to be appraised.
Dynamic Vapor Sorption (DVS) instruments are commonly used to measure sorption isotherms on a diverse array of materials, including powders, fibers, films, particulates, and other solid surfaces.
These isotherms can be obtained with various gases and vapors over a wide range of temperatures. Isotherm size, shape and hysteresis can elucidate mechanistic information about vapor/gas-solid interactions. Further, by applying the correct isotherm models, it is possible to determine additional material properties, such as surface areas, monolayer capacities, micro- and meso-porosity, and sorption enthalpies. This educational and informational webinar focuses on the interpretation of isotherm attributes and the application of various surface and bulk isotherm models.
In the real world, capture/adsorption of CO2 is often a competition with other gas phase species, most commonly water vapour. In some cases, the concentration of H20 vapor is much higher than that of CO2 further complicating the adsorption process. This presentation compares the use of two of the most commonly used experimental approach for studying CO2 sorption in the presence of H20 vapor; gravimetric analysis using Dynamic Vapour Sorption (DVS) and breakthrough analyser studies. Both experimental details and case study comparisons of adsorbent performance will be presented in this webinar.
Sorption techniques have been used to study the state of water molecules and their role in physicochemical stability of pharmaceutical and food materials. The sorption data has been used to measure the surface area and activation energy of diffusion for as received and modified pharmaceutical ingredients. Change in the temperature significantly influences the amount of the sorbed and desorbed water. The overall conclusion could be translated in the drying phase of the final pharmaceutical formulation.
The webinar presented the theory and calculation process of the BET Specific Surface Area (SSA) and Surface Energy (SE) analysis using inverse Gas Chromatography (iGC) and the comparison with other techniques.
Inverse Gas Chromatography can be used to measure both infinite and finite dilution experiments depending on the requirement of the application.
BET SSA analysis is a finite dilution experiment requiring saturation to monolayer coverage of the surface with the vapor phase probe molecules and where solute-solute interactions can occur. Conversely, surface energy analysis requires infinite dilution where only solute-solid material interaction occurs.