Heriot Watt University Team Wins Prestigious Carbon Capture Innovation Prize
Singapore, July 19, 2024 — In a significant stride towards addressing climate change, the Carbon Capture Innovation Prize has recognized pioneering efforts in the emerging field of carbon capture. This prestigious competition, organized in conjunction with the MOF2024 conference, underscores the critical importance of developing sustainable materials solutions to combat global warming.


The Competition: Accelerating Climate Solutions
The Carbon Capture Innovation Prize, organized by Surface Measurement Systems (SMS), was a groundbreaking contest aimed at accelerating the development of innovative carbon capture technologies. Held alongside the MOF2024 conference in Singapore in July, the competition focused on the innovative use of a state-of-the-art scientific instrument designed to measure the capture of carbon dioxide in adsorbent materials. The challenge invited startups, SMEs, and academic institutions to present their cutting-edge research on both direct air capture and flue gas capture technologies.
A Reward for Innovation
The winner of this esteemed contest receives a DVS Carbon instrument, valued at over $150,000, provided free of charge to their institution. The DVS Carbon is a pioneering sorption analyzer designed for advanced carbon capture, utilization, and storage (CCUS) applications. Equipped with Surface Measurement Systems’ patented Ultrabalance technology, the DVS Carbon enables real-time mass change measurements during sorption and desorption processes, facilitating the assessment of complex carbon capture conditions across various materials. This powerful tool, with its high-precision control of temperature and humidity over a broad range of CO₂ concentrations, will enable the winning team to advance their research, significantly contributing to the global effort to reduce carbon emissions and fight global warming.
“We launched this competition to recognize and reward outstanding research in carbon capture materials. By providing advanced tools and fostering collaboration, we aim to accelerate breakthroughs that are vital for mitigating climate change.”
– Prof. Daryl Williams, MD & Founder, Surface Measurement Systems
Rigorous Evaluation and Submissions
| The competition attracted over 90 proposals from researchers worldwide. Each submission underwent rigorous evaluation by a panel of expert judges, who assessed the proposals based on the following criteria:
1. Relevance to Carbon Capture: Alignment with the overarching goals of developing efficient CO2 capture technologies. 2. Innovation: Novelty and originality of the research approach. 3. Scientific Rigor: Validity and robustness of the proposed methodology. 4. Impact Potential: Potential contributions to advancing carbon capture research. 5. Clarity and Feasibility: Coherence and realism of the research plan. 6. Potential for Collaboration: Openness to collaboration and knowledge exchange within the scientific community. The judges sought proposals that addressed the challenges and opportunities in carbon capture, demonstrated innovative ideas, and exhibited sound scientific methodologies. The potential impact of the research on advancing the field, as well as the clarity and feasibility of the proposed plans, were crucial evaluation factors. Proposals that highlighted a pathway to new fundamental understanding, novel materials, or solutions to process scale-up challenges were particularly valued. |

Finalists and the Ultimate Winner
After a meticulous review, the judges selected six finalists:
- Arizona State University (USA)
- Avnos (USA)
- CICECO – Aveiro Institute of Materials (Portugal)
- Georgia Institute of Technology (USA)
- Heriot-Watt University (UK)
- Tsinghua University (China)
Finally, in an award ceremony during the closing sessions of MOF2024, Prof Susana Garcia and her team from Heriot-Watt University in the UK were announced as the winners of the Carbon Capture Innovation Prize. Prof Garcia expressed her gratitude and excitement:
“I am extremely honoured to receive the prestigious SMS 2024 Carbon Capture Innovation Prize, which recognises our efforts and pioneering work on accelerating carbon capture technologies. We aim to accelerate the transition to a Net-Zero carbon economy by developing an open-access experimental protocol, which accurately characterises the thermodynamics, in-depth mass-transfer mechanisms and CO2-H2O adsorption kinetics on Direct Air Capture (DAC) sorbent materials using the unique features of the DVS Carbon. It will address knowledge gaps in adsorption kinetic studies for solid sorbents under realistic conditions, providing essential, rigorously produced data. This data will aid in assessing and designing sorbents for optimal DAC process performance and standardising measurement protocols for benchmarking within the carbon capture community.”– Prof Susana Garcia, Heriot-Watt University

A Brighter Future for Carbon Capture
Looking ahead, Surface Measurement Systems remains committed to supporting Prof. Garcia and her team in their research endeavors. Dr. Williams expressed his enthusiasm for the partnership, stating, “We eagerly anticipate the advancements that Prof. Garcia and her team will achieve with the DVS Carbon instrument. Their work exemplifies the innovative spirit and scientific excellence that this competition aims to promote. We look forward to sharing their progress and its impact on a more carbon-efficient future.”
For more information on the Carbon Capture Innovation Prize and updates on the winner’s research, check back soon at www.sorptionhub.com. You can find out more about the DVS Carbon instrument here.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.

Gravimetric |
Volumetric |
Chromatographic |
|
Short Description |
A microbalance precisely measures the change in sample weight as gas or vapor is adsorbed, offering direct and accurate uptake data. |
The sample is placed in a sealed cell where controlled gas doses are introduced. Uptake is calculated by monitoring pressure changes over time. |
A gas stream of known flow rate passes through a packed bed of sample. Changes in outlet concentration are used to determine sorption via mass balance. |
Primary Measured Parameters |
Sample weight change. Temperature & pressure/concentration. |
Pressure decay in a closed cell of known volume. Temperature & pressure/concentration. |
Flowrate in/out of a packed bed. Temperature & pressure/concentration before and after the bed. |
Sample Minimum Amount |
from 1-10 mg |
from ~50-100 mg |
From 50-100 mg |
Sample Maximum Amount |
1 – 100 g |
1-5 g |
100 g – few kg |
Pros |
Highly accurate and flexible with samples and measurement conditions.Small sample amounts needed.Direct measurement of uptake.Can operate in both dynamic and static mode.Provides sorption kinetics by default.Can easily perform both isotherm (pressure scan) and isobar (temperature scan) experiments.Sample drying/activation is followed directly and quantified. |
Low complexity, simple to implement and to parallelize.Straightforward to obtain wide temperature and pressure ranges.Easier to hyphenate as the sample can be located away from the instrument. |
Provides true multicomponent sorption data from mixtures.Pulse retention time can be used to determine isotherms and surface parameters.IGC method can measure minute surface areas.Can provide information into process relevant parameters – mass and heat transfer. |
Cons |
Complex engineering required– best with bespoke instrumentation.Cannot measure true multicomponent sorption, though can measure total amount adsorbed from a mixture.Buoyancy correction may be difficult at high pressures. |
Indirect measurement of uptake – must rely on equations of state.Cannot use dynamic or carrier probe introduction.
|
Indirect measurement of uptake – must rely on equations of state.Complex to set up and correctly analyze data – best with bespoke instrumentation.Often not suitable for fine powders due to pressure drop. |
Method Applicable |
Static & dynamic introductionPure & carrier |
Static onlyPure & carrier |
Dynamic onlyPure & carrier |

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.



Gravimetric Method
Gravimetric techniques determine gas or vapor uptake by directly measuring changes in sample weight using a highly sensitive microbalance. As conditions such as temperature and pressure or vapor concentration change, the balance detects shifts in net force, which correspond to the amount of sorbate absorbed or adsorbed by the material.
Because they rely on precise weight measurements, gravimetric methods such as Dynamic Vapor Sorption (DVS) are considered among the most accurate techniques for sorption analysis. They are especially useful for studying a wide range of materials under controlled and variable environmental conditions.
| Sample Mass Uptake
In a standard experiment, the sample is loaded in the balance and isolated in a chamber. The chamber temperature is carefully monitored, alongside the pressure/concentration of the gas phase. Change in mass given by the balance has three contributions: the change in sample mass given by uptake in the sample, a change in net buoyant force given by the change in density of the gas phase and a drag force of a gas flow on the sample pan. Buoyancy Force The buoyancy force is small for experiments at ambient pressure and temperature but can play a role at high pressure (>a few bar) where corrections may be required. Drag Force The drag force is only relevant when a dynamic mode is used, as the gas flow impinges on the balance parts and may need to be corrected through a blank. A way of minimizing both drag and buoyancy corrections is to construct the balance symmetrically, having a sample and a reference pan in identical configurations in the flow path. Experimental Design
Advantages of Gravimetric Methods One of the key advantages of gravimetric sorption methods is their flexibility in sample size. For single-component measurements, as little as 1–5 mg of material is often sufficient to yield highly reliable results. At the same time, gravimetric systems can accommodate much larger sample sizes, handling several grams or tens of cubic centimeters, depending on the material and application. The accuracy and range of measurements are directly tied to the performance and stability of the microbalance. For example, Surface Measurement Systems (SMS) offers advanced gravimetric analyzers with exceptionally high resolution:
This combination of precision and versatility makes gravimetric methods ideal for both delicate, small-scale experiments and larger, industrially relevant studies. Challenges and Considerations While gravimetric sorption techniques are known for their high sensitivity and accuracy, there are a few practical considerations to keep in mind. Gravimetric systems typically require a stable laboratory environment, particularly with respect to temperature and vibration control, to ensure consistent results. Additionally, extended equilibration times may be needed for certain materials or conditions, especially when studying slow diffusion processes. |

Volumetric Method
In volumetric methods, a cell containing the adsorbent material is dosed sequentially from a known volume of gas. The pressure is monitored as the probe sorbs into the material, and the equilibrium pressure is then used to calculate the final amount of gas remaining in the cell using an equation of state. The difference between the initial and final amounts of gas is equal to the amount adsorbed.
Sample Uptake
Advantages of Volumetric Methods
Challenges and Considerations Volumetric sorption techniques rely on indirect measurement via pressure, which introduces several important limitations:
|

Chromatographic / Breakthrough Methods
In chromatographic methods, the probe molecule flows through a packed bed or column containing the material to be characterized. Key variables including temperature, total pressure or concentration, and flow rate are continuously monitored at both the inlet and outlet of the column. These measurements allow for the calculation of a molar mass balance across the packed bed, providing insight into the sorption behavior of the material under dynamic flow conditions.
| Method Modes Chromatographic and breakthrough methods are dynamic flow techniques used to study how probe molecules interact with packed columns. Most systems operate in a carrier mode for easier pressure control. Probe introduction typically happens in two ways:
Experimental Process A sample-packed column is weighed before and after preparation, with thermal activation applied if needed. The system’s dead volume is measured using a non-interacting gas pulse. The probe pulse or front passes through the column, and sensors measure outlet concentration and flow to determine uptake via mass balance. Challenges and Considerations Controlling temperature, pressure, concentration, and flow precisely makes this method complex. It requires larger sample amounts (hundreds of mg to grams) and can be scaled for industrial simulation. Sample form affects performance, as fine powders may cause blockages. Additional Capabilities These methods uniquely provide multicomponent adsorption data and insight into adsorption kinetics critical for scaling processes. Surface Measurement Systems offers the iGC-SEA and BTA Frontier analyzers as leading tools for these analyses.
|

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.


Gravimetric Method
Gravimetric techniques determine gas or vapor uptake by directly measuring changes in sample weight using a highly sensitive microbalance. As conditions such as temperature and pressure or vapor concentration change, the balance detects shifts in net force, which correspond to the amount of sorbate absorbed or adsorbed by the material.
Because they rely on precise weight measurements, gravimetric methods such as Dynamic Vapor Sorption (DVS) are considered among the most accurate techniques for sorption analysis. They are especially useful for studying a wide range of materials under controlled and variable environmental conditions.
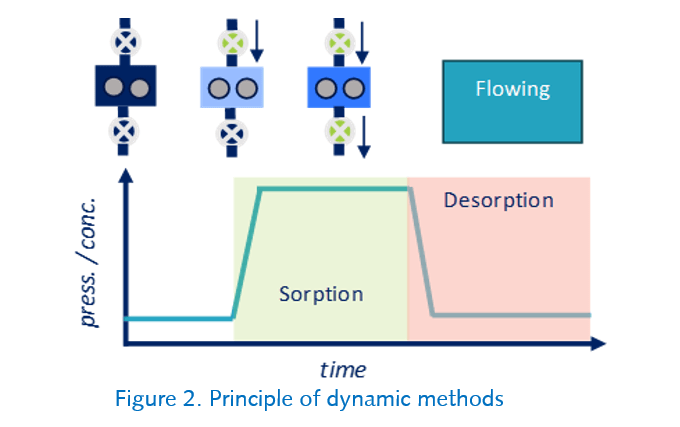
Dynamic (Flowing)
In the dynamic method, a continuous flow of gas or vapor is passed over the sample. The method controls both the upstream rates of entry and the downstream exit rate, effectively controlling both the pressure/concentration and flow rate of the adsorbate in the chamber. It also helps to overcome some boundary layer diffusional barriers, by ensuring little to no concentration gradients around the sample. Both pure and carrier introduction is possible.
Note: The SMS DVS, SMS iGC-SEA and SMS BTA systems operate in dynamic mode, while the SMS DVS Vacuum can perform both static and dynamic introduction.

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.


Gravimetric Method
Gravimetric techniques determine gas or vapor uptake by directly measuring changes in sample weight using a highly sensitive microbalance. As conditions such as temperature and pressure or vapor concentration change, the balance detects shifts in net force, which correspond to the amount of sorbate absorbed or adsorbed by the material.
Because they rely on precise weight measurements, gravimetric methods such as Dynamic Vapor Sorption (DVS) are considered among the most accurate techniques for sorption analysis. They are especially useful for studying a wide range of materials under controlled and variable environmental conditions.
Do the results differ?
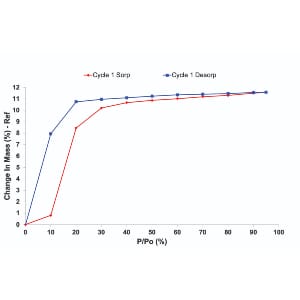
Interestingly, the number of probe molecules that interact with the material is the same in both methods. As illustrated in Figure 2, the equilibrium uptake is typically similar, regardless of the introduction method. However, the kinetics of sorption can vary, with carrier gas mode often offering a more accurate picture of how materials behave under practical conditions.
It’s important to note that for certain materials, even the choice of carrier gas may impact sorption behavior, so careful selection is essential.
Which Method Should You Use?
Both techniques are powerful tools in surface and materials science. The choice depends on your experimental goals:
- Choose pure mode when maximum control and absolute data are required.
- Opt for carrier mode when simulating realistic environments or studying sorption kinetics

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.

The Role of Temperature in SorptionThe average kinetic energy of all molecules in the system is unambiguously defined by temperature. Temperature is represented in Kelvin (K, the SI unit), degree Celsius (°C) or degree Fahrenheit (°F). Although measuring temperature accurately and ensuring its homogeneity throughout an instrument can be challenging, this discussion will focus on the relationship between temperature and kinetic energy. On the other hand, the number of target probe molecules which interact with the material at any point can be reported in a variety of ways, with terms such as pressure, absolute/partial pressure, relative pressure, humidity, water activity, volume/molar fraction and concentration, etc. The variety of terms will be discussed in more detail. |
Understanding Pressure and its VariantsAbsolute Pressure Total and Partial Pressure Fraction and Concentration One further complication is that the fraction can be either on a molar, volume, or mass basis of the components. The best way to represent the meaning of a fraction basis is through an example. In a balloon we put one mole of N2 and one mole of CO2. The molar fraction of N2 is 1/(1+1) = 0.5 in this mixture. To get the volume fraction, we must instead consider the volume contribution of each gas. If we consider both gases as ideal, a mole of each occupies 22.4 L, so for N2 the volume fraction is 22.4/(22.4+22.4) = 0.5. Finally for the mass fraction, we need to convert to weight by using the molar mass of each component, resulting in 28g of N2 and 44g of CO2. The mass fraction of N2 is then 28/(28+44) = 0.388. To differentiate between the three, we often use notation such as mol%, vol% and wt%. Sometimes fractions are also expressed as parts of a whole, e.g. parts-per-million or ppm. These can be converted into a regular fraction by dividing by the total number of parts. It is also important to refer to which basis these refer to, by using notation such as ppmv or ppmw, etc. These units are generally discouraged as they are confusing. Relative Pressure, Humidity and Water Activity |
|
| The formula for relative pressure is |
| Relative pressure is usually represented with the shorthand p/p0, ranging from 0 to 1, or from 0 to 100% if using a percent value. Humidity (RH) or water activity are both relative pressure terms, specifically used when the vapor is water. |

Critical Points and Relative Pressure
Above the critical point of a compound, there is no distinction between a liquid and gas state, which means that there is no vapor pressure. Such gases (for example N2 and CH4 at room temperature) cannot be represented in a relative pressure.
Figure 1 summarizes the different ways or reporting total pressure.

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.

The Role of Temperature in SorptionThe average kinetic energy of all molecules in the system is unambiguously defined by temperature. Temperature is represented in Kelvin (K, the SI unit), degree Celsius (°C) or degree Fahrenheit (°F). Although measuring temperature accurately and ensuring its homogeneity throughout an instrument can be challenging, this discussion will focus on the relationship between temperature and kinetic energy. On the other hand, the number of target probe molecules which interact with the material at any point can be reported in a variety of ways, with terms such as pressure, absolute/partial pressure, relative pressure, humidity, water activity, volume/molar fraction and concentration, etc. The variety of terms will be discussed in more detail. |

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Unlocking Surface Energy Insights with the iGC-SEA Nova
iGC-SEA Nova Case Study


The 2024 Carbon Capture Innovation Prize Launches:
A New Research Competition
Singapore, April 2024 | Surface Measurement Systems is thrilled to announce the launch of the 2024 Carbon Capture Innovation Prize, a prestigious competition designed to accelerate research in the field of carbon capture technology. In a groundbreaking initiative alongside MOF2024, this challenge aims to support researchers seeking to revolutionize the way we employ materials to combat carbon emissions via carbon capture, and to highlight their pioneering research work. The final winner of this Prize will be awarded a DVS Carbon instrument valued at $150,000, a state-of-the-art scientific instrument that accurately measures CO2 uptake in materials under a range of real-world conditions.

| Fostering Innovation and Sustainability
As the world grapples with the escalating challenges of climate change, the need for innovative and sustainable solutions has never been more critical. The 2024 Carbon Capture Innovation Prize aims to harness the power of scientific ingenuity to develop new materials and technologies capable of capturing CO2 efficiently from atmospheric and industrial sources, thus contributing to a more carbon-neutral society. Surface Measurement Systems is committed to supporting global researchers who working on this important topic. About the Challenge Run in coordination with the world leading MOF2024, taking place 15 – 19 July 2024 in Singapore, this challenge invites professionals, academics, and innovators to submit a brief proposal on how a DVS Carbon would help their research ambitions. This state-of-the-art sorption analyzer is designed for precise measurement of CO2 capture in solid state materials across various test conditions. Competition entrants will submit a short research proposal on how they propose to employ this instrument to investigate new and exciting possibilities in materials for carbon capture. A panel of 5 international expert judges will evaluate the research proposals submitted to determine a final shortlist, and the winner. Eligibility and Awards The contest is open to professional researchers (no student applicants) affiliated with academic institutions, government laboratories, or startup companies. Entrants must have the authority to install the awarded instrument in their designated research area/laboratory. The winning submission will be awarded a DVS Carbon instrument, valued at over $150,000, to significantly bolster their research capabilities in discovering new materials for CO2 capture. The DVS Carbon The latest instrument in Surface Measurement Systems’ Dynamic Vapor Sorption series, the DVS Carbon sets a new industry benchmark for evaluating solid-state materials for CO2 capture. Capable of measuring uptake at a wide range of CO2 concentrations, temperatures, and in the presence of moisture, the DVS Carbon opens up exciting possibilities for predicting performance in new adsorbent materials for use in the CCUS space. This award aims to get the research community thinking about what they could achieve with this instrument, and provide a gateway for them to get supporting in achieving their goals. Submission Guidelines and Evaluation Criteria Proposals must be comprehensive, detailing the team’s background, research objectives, methodology, and the potential impact of their work on climate change solutions. Submissions will be evaluated based on innovation, scientific rigor, impact potential, and clarity. A panel of independent reviewers will select the winner and runners-up, with a preference for early career researchers in the event of a competition tie. An application form is available here Recognition and Collaboration The winner and four runners-up will be showcased at an official ceremony during MOF2024 conference , gaining substantial exposure among leading researchers. Additionally, the winner will have the opportunity to share their findings in future webinars hosted by Surface Measurement Systems, fostering collaboration and knowledge exchange within the scientific community. Join the Movement Towards a Carbon-Neutral Future Surface Measurement Systems invites global researchers to partake in this unique opportunity to contribute to a more sustainable future, and secure a hugely valuable research tool for developing advanced material solutions. The deadline for submissions is June 17th, 2024, with the winner to be announced during the MOF2024 conference. For more information on the Carbon Capture Innovation Challenge and how to submit your proposal, please visit www.sorptionhub.com/mof24-cc-prize |

About Surface Measurement Systems
Surface Measurement Systems is at the forefront of developing innovative technologies for material science. Specializing in advanced analytical instruments, the company is dedicated to supporting research that addresses some of the most pressing environmental challenges of our time.
Join us in shaping the future of carbon capture technology and making a tangible impact on reducing global warming.

Unlocking Surface Energy Insights with the iGC-SEA Nova
iGC-SEA Nova Case Study