Across the World,
SMS’ers Celebrated the Festivities in Style!
It’s been a busy end to the year for Surface Measurement Systems, with a huge push in instrument sales, Production sending products flying out the door, and Sales still hitting the road to drum up those last bits of busy!
Somehow, we’ve still found time across the world to celebrate our festive seasons as a team! From Diwali in Hyderabad to Christmas in London, the Global SMS Team celebrated the festivities in style!

SMS India & PCL Celebrate Diwali
| The SMS India & PCL Team held a special event to celebrate the five-day Diwali Festival. Marking the Hindu New Year, Diwali symbolizing new beginnings and the triumph of good over evil. Also known as the Festival of Lights, Diwali is celebrated through the sharing of meals and sweets, decorating with oil lamps called “diyas”, making colorful patterns on the floor called rangoli (see below), and setting off fireworks. Hopefully, this last activity was done outside of the office.
Happy Diwali to all our Hindu colleagues in Hyderabad, and around the world! |
SMS UK Toast Christmas at Pub Party
| The team from Surface Measurement Systems London got in the Festive spirit with a company lunch at the Defector’s Weld Pub in Shepherd’s Bush. Following a hearty meal, company Founder & MD, assumed the sacred duty of handing out Secret Santa gifts to all members of staff. |

SMSNA Saving the Best for Last
Not to be outdone, our colleagues at SMSNA are saving their best for last, having their Christmas Staff Meal later this week! We wish them a happy Season’s Greetings, and hope they have a great day celebrating the festive season!

Carbon Capture
Sorption Science Pack
Download Free Now
Surface Measurement Systems have been pioneering sorption-based solutions Carbon Capture, developing groundbreaking new instruments that enable analysis and insights never possible before.
Please download this free pack of recent scientific publications on Carbon Capture Applications of sorption science, including recent case studies and scientific overviews. If you have any questions about the topics explored, please do stop by our stand at AiCHE 2024.
Expanding the Senior Leadership Team:
Driving the Future of
Surface Measurement Systems
Surface Measurement Systems Holding LLC and its subsidiaries are entering an exciting new chapter, marked by growth, innovation, and an ambitious vision for the future. To support this next phase, we have expanded our Senior Leadership Team, ensuring we have the expertise and leadership needed to meet our goals.
This expansion includes elevating talented team members and welcoming new leaders, bringing fresh perspectives and expertise. With this enhanced team in place, SMS is better equipped than ever to drive innovation, seize emerging business opportunities, and continue improving our solutions to meet the evolving needs of our customers.
The future of Surface Measurement Systems is bright, and this leadership expansion reflects our commitment to staying at the forefront of sorption innovation, delivering exceptional value and results.
Senior Leadership Team

Europe & US Symposiums bring leading sorption scientist together
2024 saw a grand return for the live editions of the Sorption Symposium Series. With the 202 Pandemic scuppering plans for a live scientific event in 2020, Surface Measurement Systems were thrilled to be able to bring the event back to the face-to-face format, enabling a shared learning and networking experience for sorption scientists from around the world.
Eager to offer an in-person return to our users and peers around the world, the Sorption Symposium saw two live events held this year. The first, taking place in May, was held at the New Jersey Institute of Technology, while the second returned to the venue we had to cancel in 2020, University of Vienna, for our European symposium in September.

Sorption Symposium North America 2024 (7 – 9 May)
Gathers Leading Sorption Experts in New Jersey
Gathers Leading Sorption Experts in New Jersey
| The Sorption Symposium North America 2024, organized by Surface Measurement Systems, recently concluded with great success, showcasing the latest advancements in sorption science. Held in Allentown, PA, the event attracted over 60 attendees from various industries, including pharmaceuticals, environmental sciences, and material engineering. It offered a rich program featuring renowned speakers such as Dr. Maria Krisch from FreeThink Technologies and Dr. Neeraj Borker from 3M. Their presentations, alongside other experts, covered critical topics in sorption behavior, moisture analysis, and its applications across diverse fields.
A highlight of the event was the strong participation in the hands-on training sessions, where attendees received practical insights and guidance from an expert panel. The training provided an interactive platform for participants to deepen their understanding of dynamic vapor sorption (DVS) technology and its role in enhancing material performance, stability, and formulation development. This engaging format fostered an environment of collaboration and learning, elevating the educational value of the symposium. The symposium also created ample opportunities for networking, particularly during the on-site Networking Party. Attendees had the chance to connect with peers, exchange ideas, and explore potential collaborations, further strengthening the event’s impact. Overall, the Sorption Symposium North America 2024 was a resounding success, bringing together a diverse group of professionals to share knowledge, engage in hands-on experiences, and build relationships within the sorption science community. |
|
 |
 |
 |
 |
University of Vienna Plays Host to
Sorption Symposium Europe 2024 (18 – 20th Sept)
Sorption Symposium Europe 2024 (18 – 20th Sept)
|
The Sorption Symposium Europe 2024, hosted by Surface Measurement Systems, was a resounding success, gathering over 70 attendees at the University of Vienna from September 18-20. This premier event brought together leading minds from academia and industry to explore cutting-edge advancements in sorption science, particularly focusing on Dynamic Vapor Sorption (DVS) and Inverse Gas Chromatography (iGC), and recent developments in Carbon Capture Sorption Analysis capabilities. The symposium featured a stellar lineup of speakers, including Dr. Daniel Burnett and Dr. Anett Kondor from Surface Measurement Systems, along with global experts like Dr. Neeraj Borker (3M), Prof. Peter Budd (UManchester), Dr. Sam Zelinka (U.S. Forest Service), and many more. These presentations covered a broad spectrum of applications in pharmaceuticals, carbon capture, and material science, offering a comprehensive look at current research and future potential. Day three saw strong participation in hands-on workshops, where attendees received in-depth training on advanced sorption instruments, gaining valuable skills from global experts. In addition to the academic content, attendees enjoyed vibrant networking events, which included a wine and beer tasting, a traditional Austrian meal, and a unique ‘Hidden Vienna’ tour. These activities allowed participants to form lasting professional relationships in a relaxed atmosphere, enhancing the collaborative spirit of the symposium. |
|
 |
 |
 |
 |

New starters & Qualifications
The team at Surface Measurement Systems continues to expand, with every office seeing new members join us for the exciting times ahead! .
Below is a quick review of who has joined us since Christmas.

New Team members

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.

Gravimetric |
Volumetric |
Chromatographic |
|
Short Description |
A microbalance precisely measures the change in sample weight as gas or vapor is adsorbed, offering direct and accurate uptake data. |
The sample is placed in a sealed cell where controlled gas doses are introduced. Uptake is calculated by monitoring pressure changes over time. |
A gas stream of known flow rate passes through a packed bed of sample. Changes in outlet concentration are used to determine sorption via mass balance. |
Primary Measured Parameters |
Sample weight change. Temperature & pressure/concentration. |
Pressure decay in a closed cell of known volume. Temperature & pressure/concentration. |
Flowrate in/out of a packed bed. Temperature & pressure/concentration before and after the bed. |
Sample Minimum Amount |
from 1-10 mg |
from ~50-100 mg |
From 50-100 mg |
Sample Maximum Amount |
1 – 100 g |
1-5 g |
100 g – few kg |
Pros |
Highly accurate and flexible with samples and measurement conditions.Small sample amounts needed.Direct measurement of uptake.Can operate in both dynamic and static mode.Provides sorption kinetics by default.Can easily perform both isotherm (pressure scan) and isobar (temperature scan) experiments.Sample drying/activation is followed directly and quantified. |
Low complexity, simple to implement and to parallelize.Straightforward to obtain wide temperature and pressure ranges.Easier to hyphenate as the sample can be located away from the instrument. |
Provides true multicomponent sorption data from mixtures.Pulse retention time can be used to determine isotherms and surface parameters.IGC method can measure minute surface areas.Can provide information into process relevant parameters – mass and heat transfer. |
Cons |
Complex engineering required– best with bespoke instrumentation.Cannot measure true multicomponent sorption, though can measure total amount adsorbed from a mixture.Buoyancy correction may be difficult at high pressures. |
Indirect measurement of uptake – must rely on equations of state.Cannot use dynamic or carrier probe introduction.
|
Indirect measurement of uptake – must rely on equations of state.Complex to set up and correctly analyze data – best with bespoke instrumentation.Often not suitable for fine powders due to pressure drop. |
Method Applicable |
Static & dynamic introductionPure & carrier |
Static onlyPure & carrier |
Dynamic onlyPure & carrier |

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.



Gravimetric Method
Gravimetric techniques determine gas or vapor uptake by directly measuring changes in sample weight using a highly sensitive microbalance. As conditions such as temperature and pressure or vapor concentration change, the balance detects shifts in net force, which correspond to the amount of sorbate absorbed or adsorbed by the material.
Because they rely on precise weight measurements, gravimetric methods such as Dynamic Vapor Sorption (DVS) are considered among the most accurate techniques for sorption analysis. They are especially useful for studying a wide range of materials under controlled and variable environmental conditions.
| Sample Mass Uptake
In a standard experiment, the sample is loaded in the balance and isolated in a chamber. The chamber temperature is carefully monitored, alongside the pressure/concentration of the gas phase. Change in mass given by the balance has three contributions: the change in sample mass given by uptake in the sample, a change in net buoyant force given by the change in density of the gas phase and a drag force of a gas flow on the sample pan. Buoyancy Force The buoyancy force is small for experiments at ambient pressure and temperature but can play a role at high pressure (>a few bar) where corrections may be required. Drag Force The drag force is only relevant when a dynamic mode is used, as the gas flow impinges on the balance parts and may need to be corrected through a blank. A way of minimizing both drag and buoyancy corrections is to construct the balance symmetrically, having a sample and a reference pan in identical configurations in the flow path. Experimental Design
Advantages of Gravimetric Methods One of the key advantages of gravimetric sorption methods is their flexibility in sample size. For single-component measurements, as little as 1–5 mg of material is often sufficient to yield highly reliable results. At the same time, gravimetric systems can accommodate much larger sample sizes, handling several grams or tens of cubic centimeters, depending on the material and application. The accuracy and range of measurements are directly tied to the performance and stability of the microbalance. For example, Surface Measurement Systems (SMS) offers advanced gravimetric analyzers with exceptionally high resolution:
This combination of precision and versatility makes gravimetric methods ideal for both delicate, small-scale experiments and larger, industrially relevant studies. Challenges and Considerations While gravimetric sorption techniques are known for their high sensitivity and accuracy, there are a few practical considerations to keep in mind. Gravimetric systems typically require a stable laboratory environment, particularly with respect to temperature and vibration control, to ensure consistent results. Additionally, extended equilibration times may be needed for certain materials or conditions, especially when studying slow diffusion processes. |

Volumetric Method
In volumetric methods, a cell containing the adsorbent material is dosed sequentially from a known volume of gas. The pressure is monitored as the probe sorbs into the material, and the equilibrium pressure is then used to calculate the final amount of gas remaining in the cell using an equation of state. The difference between the initial and final amounts of gas is equal to the amount adsorbed.
Sample Uptake
Advantages of Volumetric Methods
Challenges and Considerations Volumetric sorption techniques rely on indirect measurement via pressure, which introduces several important limitations:
|

Chromatographic / Breakthrough Methods
In chromatographic methods, the probe molecule flows through a packed bed or column containing the material to be characterized. Key variables including temperature, total pressure or concentration, and flow rate are continuously monitored at both the inlet and outlet of the column. These measurements allow for the calculation of a molar mass balance across the packed bed, providing insight into the sorption behavior of the material under dynamic flow conditions.
| Method Modes Chromatographic and breakthrough methods are dynamic flow techniques used to study how probe molecules interact with packed columns. Most systems operate in a carrier mode for easier pressure control. Probe introduction typically happens in two ways:
Experimental Process A sample-packed column is weighed before and after preparation, with thermal activation applied if needed. The system’s dead volume is measured using a non-interacting gas pulse. The probe pulse or front passes through the column, and sensors measure outlet concentration and flow to determine uptake via mass balance. Challenges and Considerations Controlling temperature, pressure, concentration, and flow precisely makes this method complex. It requires larger sample amounts (hundreds of mg to grams) and can be scaled for industrial simulation. Sample form affects performance, as fine powders may cause blockages. Additional Capabilities These methods uniquely provide multicomponent adsorption data and insight into adsorption kinetics critical for scaling processes. Surface Measurement Systems offers the iGC-SEA and BTA Frontier analyzers as leading tools for these analyses.
|

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.


Gravimetric Method
Gravimetric techniques determine gas or vapor uptake by directly measuring changes in sample weight using a highly sensitive microbalance. As conditions such as temperature and pressure or vapor concentration change, the balance detects shifts in net force, which correspond to the amount of sorbate absorbed or adsorbed by the material.
Because they rely on precise weight measurements, gravimetric methods such as Dynamic Vapor Sorption (DVS) are considered among the most accurate techniques for sorption analysis. They are especially useful for studying a wide range of materials under controlled and variable environmental conditions.
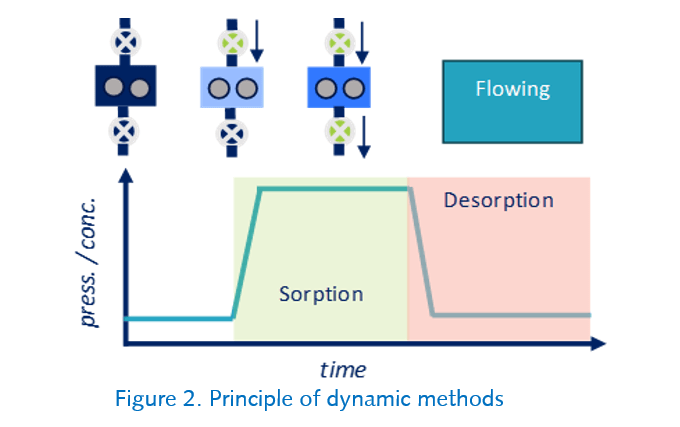
Dynamic (Flowing)
In the dynamic method, a continuous flow of gas or vapor is passed over the sample. The method controls both the upstream rates of entry and the downstream exit rate, effectively controlling both the pressure/concentration and flow rate of the adsorbate in the chamber. It also helps to overcome some boundary layer diffusional barriers, by ensuring little to no concentration gradients around the sample. Both pure and carrier introduction is possible.
Note: The SMS DVS, SMS iGC-SEA and SMS BTA systems operate in dynamic mode, while the SMS DVS Vacuum can perform both static and dynamic introduction.

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.


Gravimetric Method
Gravimetric techniques determine gas or vapor uptake by directly measuring changes in sample weight using a highly sensitive microbalance. As conditions such as temperature and pressure or vapor concentration change, the balance detects shifts in net force, which correspond to the amount of sorbate absorbed or adsorbed by the material.
Because they rely on precise weight measurements, gravimetric methods such as Dynamic Vapor Sorption (DVS) are considered among the most accurate techniques for sorption analysis. They are especially useful for studying a wide range of materials under controlled and variable environmental conditions.
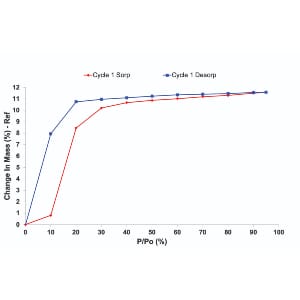
Do the results differ?
Interestingly, the number of probe molecules that interact with the material is the same in both methods. As illustrated in Figure 2, the equilibrium uptake is typically similar, regardless of the introduction method. However, the kinetics of sorption can vary, with carrier gas mode often offering a more accurate picture of how materials behave under practical conditions.
It’s important to note that for certain materials, even the choice of carrier gas may impact sorption behavior, so careful selection is essential.
Which Method Should You Use?
Both techniques are powerful tools in surface and materials science. The choice depends on your experimental goals:
- Choose pure mode when maximum control and absolute data are required.
- Opt for carrier mode when simulating realistic environments or studying sorption kinetics

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.

Sorption Overview Series #6
Demystifying Sorption Methods: A Quick Comparison for Better Research Decisions
As we conclude our ‘Sorption Overview’ series, we’ve examined the key experimental techniques used to measure gas and vapor sorption: gravimetric, volumetric, and chromatographic methods. Each technique provides valuable insights into fundamental material properties and helps evaluate material performance across a wide range of applications.
Refer to the comparison chart below to help determine which method best suits your research needs.

The Role of Temperature in SorptionThe average kinetic energy of all molecules in the system is unambiguously defined by temperature. Temperature is represented in Kelvin (K, the SI unit), degree Celsius (°C) or degree Fahrenheit (°F). Although measuring temperature accurately and ensuring its homogeneity throughout an instrument can be challenging, this discussion will focus on the relationship between temperature and kinetic energy. On the other hand, the number of target probe molecules which interact with the material at any point can be reported in a variety of ways, with terms such as pressure, absolute/partial pressure, relative pressure, humidity, water activity, volume/molar fraction and concentration, etc. The variety of terms will be discussed in more detail. |
Understanding Pressure and its VariantsAbsolute Pressure Total and Partial Pressure Fraction and Concentration One further complication is that the fraction can be either on a molar, volume, or mass basis of the components. The best way to represent the meaning of a fraction basis is through an example. In a balloon we put one mole of N2 and one mole of CO2. The molar fraction of N2 is 1/(1+1) = 0.5 in this mixture. To get the volume fraction, we must instead consider the volume contribution of each gas. If we consider both gases as ideal, a mole of each occupies 22.4 L, so for N2 the volume fraction is 22.4/(22.4+22.4) = 0.5. Finally for the mass fraction, we need to convert to weight by using the molar mass of each component, resulting in 28g of N2 and 44g of CO2. The mass fraction of N2 is then 28/(28+44) = 0.388. To differentiate between the three, we often use notation such as mol%, vol% and wt%. Sometimes fractions are also expressed as parts of a whole, e.g. parts-per-million or ppm. These can be converted into a regular fraction by dividing by the total number of parts. It is also important to refer to which basis these refer to, by using notation such as ppmv or ppmw, etc. These units are generally discouraged as they are confusing. Relative Pressure, Humidity and Water Activity |
|
| The formula for relative pressure is |
| Relative pressure is usually represented with the shorthand p/p0, ranging from 0 to 1, or from 0 to 100% if using a percent value. Humidity (RH) or water activity are both relative pressure terms, specifically used when the vapor is water. |

Critical Points and Relative Pressure
Above the critical point of a compound, there is no distinction between a liquid and gas state, which means that there is no vapor pressure. Such gases (for example N2 and CH4 at room temperature) cannot be represented in a relative pressure.
Figure 1 summarizes the different ways or reporting total pressure.

Interested in learning more about gravimetric and chromatographic techniques?
Join our Sorption Hub to gain access to a range of free educational videos from experts in sorption science or view our products.