Tackling the Top 5 Stability Challenges in Drug Development
In drug development, stability isn’t just a checkpoint, it’s the foundation of whether a therapy makes it to patients. A single instability issue can set a program back months or even years.
We’ve gathered the five of the most common risks and how scientists are addressing them.

5 Factors That Impact Drug Stability

1. Solubility and Bioavailability
What is the Risk?
Poor solubility and low permeability in active pharmaceutical ingredients (APIs), especially in BCS Class II and IV drugs, can hinder the body’s ability to absorb the drug properly.
Why it Matters?
Solubility is essential for drug success. If left unaddressed it can cause a delay in development and potential failure in preclinical or clinical stages.
Innovative Strategies at Work
Implementing advanced formulation strategies including solid dispersion, amorphous transformation and active polymorph screening help identify the most stable and bioavailable forms earlier in development. In the early stages of drug development, predictive solubility modeling helps researchers understand how a molecule behaves under specific conditions, leading to better earlier formulation decisions.
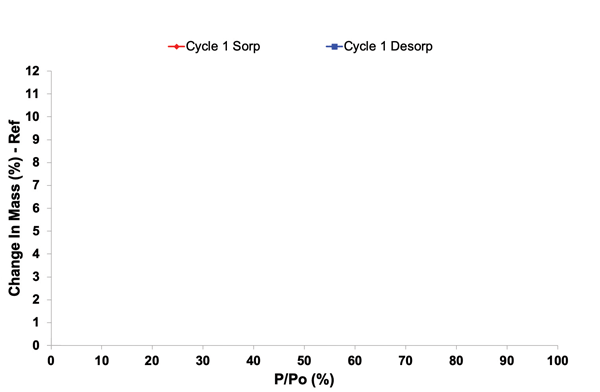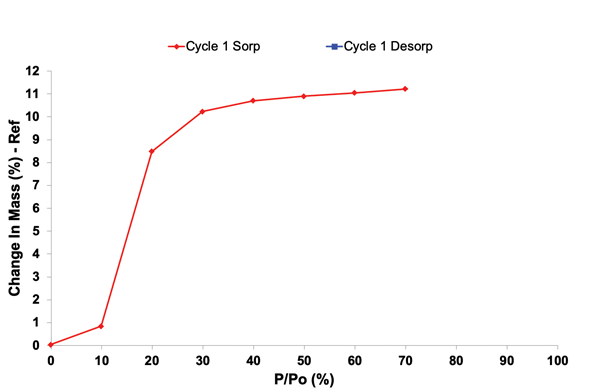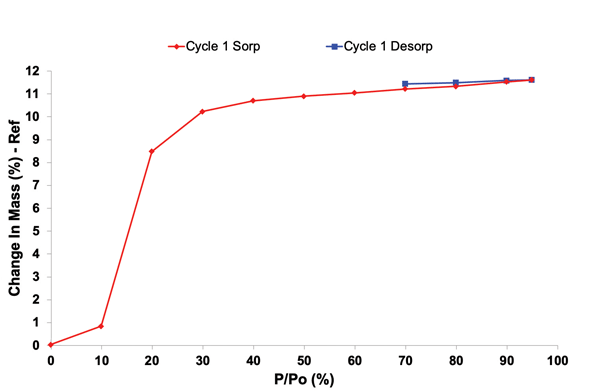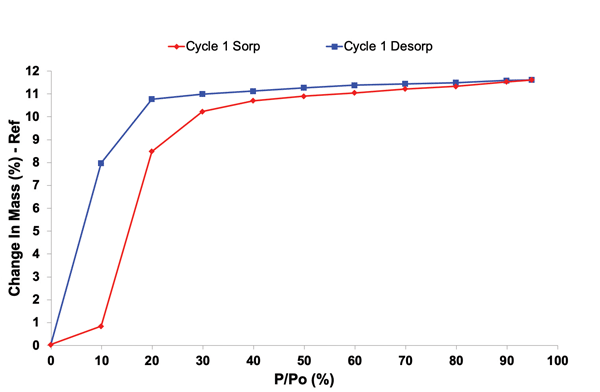
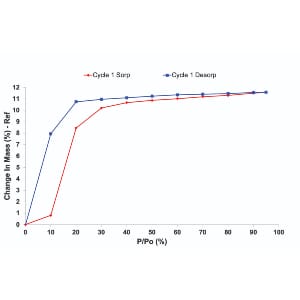
2. Environmental Conditions
What is the Risk?
Moisture, heat, and oxygen can degrade API and excipients causing chemical changes, like hydrolysis, due to moisture absorption from the environment. They can also cause polymorphic transitions can occur during storing and processing. Predicting shelf life is difficult without thorough stability analysis.
Why it Matters?
Environmental destabilization is one of the leading causes of formulation failure affecting the product’s safety, potency, and regulatory compliance which could lead to recalls or reformulation.
Innovative Strategies at Work
Stability profiling under controlled temperature and humidity conditions helps predict shelf life, guide packaging decisions, and design formulations that withstand real-world conditions. Advanced solid-state tools that provide insights into stability profiling, include Dynamic Vapor Sorption and Inverse Gas Chromatography (iGC). They can provide insights into how materials respond including adsorption kinetics, surface energy, and surface morphology.

3. Drug-Excipient Interactions
What is the Risk?
Excipients are often considered “inactive” ingredients, but their interactions with APIs could affect a drug’s stability by modifying its release profile or therapeutic effectiveness. Choosing an excipient that enhances solubility and without causing degradation can be difficult in early development stages.
Why it Matters?
Incompatible excipients can sabotage otherwise strong formulations wasting time, money, and also increases chances of formulation failure in late stages.
Innovative Strategies at Work
Compatibility studies, using tools like calorimetry or spectroscopy is standard and techniques like Inverse Gas Chromatography (iGC) help detect hidden incompatibilities early. Using the iGC to understand moisture affinity, surface chemistry, and reactivity, helps formulators select excipients that enhance performance without compromising stability.

4. Process and Scale-up Challenges
What is the Risk?
Success in lab research doesn’t always translate to successful full scale manufacturing. An increase in production volume can cause changes in granulation, drying temperature, and mixing strength. These issues can lead to powder flow issues, poor compressibility, and batch-to-batch inconsistency.
Why it Matters?
Scale-up challenges can result in production halts, corrupted test batches and regulatory delays if processes don’t result in consistent and reproducible results.
Innovative Strategies at Work
Quality by Design (QbD) and Process Analytical Technology (PAT) approaches help define critical material attributes and process parameters. Powder flow testing and material characterization ensure scalability before full-scale production. These insights allow teams to anticipate processing behavior, adjust conditions early, and avoid costly last-minute rework during scale-up.

5.Moisture and Sorption Behavior
What is the Risk?
APIs and excipients are moisture sensitive, which can lead to crystallization, amorphization, or chemical degradation. Precise models of sorption behavior is essential to accurately predict how these changes will impact a drug’s stability, performance, and shelf life.
Why it Matters?
Uncontrolled moisture uptake can reduce product shelf-life resulting in either over-engineering or under-protecting the drug.
Innovative Strategies at Work
Humidity-controlled studies and sorption profiling reveal how APIs and excipients interact with water vapor. When combined with techniques like iGC, formulators gain a complete picture of moisture behavior, packaging needs, and long-term stability.

The Role of Regulatory Guidelines in Drug Stability
Across all these risks, stability testing is guided by international standards from the FDA, EMA, NMPA, and Pharmacopeias (USP, Ph. Eur., ChP). Compliance ensures stability data is valid, comparable, and accepted globally, paving the way for faster approvals and safer launches.

What can you do?
Stability challenges are a constant in pharmaceutical development, but with the right strategies, they don’t have to become roadblocks.
By combining predictive testing, advanced analytical tools, and smart formulation design, scientists can anticipate risks early, protect their compounds, and ultimately bring safer, more effective therapies to patients faster.
Check out our case study on pharmaceutical solid-state stability using DVS and IGC-SEA to analyze polymorphs, amorphous content, moisture sorption, and glass transition for more insights.

Sorption Science Pharma Reading Pack
Formulation, Stability, & Beyond


2024: New starters
for the SMS Team
The team at Surface Measurement Systems continues to expand, with every office seeing new members join us for the exciting times ahead!
Below is a quick review of who has joined us in the last year.

New Team members

Sorption Science Pharma Reading Pack
Formulation, Stability, & Beyond


Sorption Bulletin
Best of 2024
We are happy to offer you this Sorption Bulletin, providing an overview of some of our most exciting recent publications, peer-reviewed papers, and interesting articles in the field of sorption science.
Discover the latest insights and technological advances in a range of applications.

SMS Scientific Notes
Application Note 112: Influence of humidity and temperature on the drying kinetics, phase composition and morphology of gypsum
Download a copy
Case Study 620: Temperature effect on surface energy of battery materials
Download a copy
Application Note 70: Investigating Uniformity of Dry Powder Inhalation Formulations using Vapour Sorption Techniques
Download a copy
Application Note 109: Studying Moisture Induced Transformations and Degradation of Pharmaceutical Ingredients Using Raman Spectroscopy Combined with Dynamic Vapor Sorption
Download a copy
Application Note 110: Analyzing Multicomponent Permeation Through Barrier Membranes Using the Membrane Permeation Analyzer (MPA Horizon)
Download a copy
Application Note 111: Realistically evaluating the capabilities of Zeolite 13X as a sorbent for Direct Air Capture in different scales
Download a copy
Additional Reading
Carbon Capture Storage Review 2024
Download a copy
Article: Innovative Approaches to Analysing Moisture Interactions in Pharmaceutical Materials with Dynamic Vapor Sorption (DVS)
Read here
Publications
Scientific Paper: MOF-based sensors for the detection of airborne α-pinene, RSC Appl. Interfaces 2024, 10.1039.D4LF00027
Read here
Book: Chapter 3: The Dynamic View: Multiscale Characterisation Techniques for Flexible Frameworks
The Dynamic View: Multiscale Characterisation Techniques for Flexible Frameworks | Flexible Metal–Organic Frameworks Structural Design, Synthesis and Properties
Books Gateway | Royal Society of Chemistry (rsc.org)

Across the World,
SMS’ers Celebrated the Festivities in Style!
It’s been a busy end to the year for Surface Measurement Systems, with a huge push in instrument sales, Production sending products flying out the door, and Sales still hitting the road to drum up those last bits of busy!
Somehow, we’ve still found time across the world to celebrate our festive seasons as a team! From Diwali in Hyderabad to Christmas in London, the Global SMS Team celebrated the festivities in style!

SMS India & PCL Celebrate Diwali
| The SMS India & PCL Team held a special event to celebrate the five-day Diwali Festival. Marking the Hindu New Year, Diwali symbolizing new beginnings and the triumph of good over evil. Also known as the Festival of Lights, Diwali is celebrated through the sharing of meals and sweets, decorating with oil lamps called “diyas”, making colorful patterns on the floor called rangoli (see below), and setting off fireworks. Hopefully, this last activity was done outside of the office.
Happy Diwali to all our Hindu colleagues in Hyderabad, and around the world! |
SMS UK Toast Christmas at Pub Party
| The team from Surface Measurement Systems London got in the Festive spirit with a company lunch at the Defector’s Weld Pub in Shepherd’s Bush. Following a hearty meal, company Founder & MD, assumed the sacred duty of handing out Secret Santa gifts to all members of staff. |

SMSNA Saving the Best for Last
Not to be outdone, our colleagues at SMSNA are saving their best for last, having their Christmas Staff Meal later this week! We wish them a happy Season’s Greetings, and hope they have a great day celebrating the festive season!

Carbon Capture
Sorption Science Pack
Download Free Now
Surface Measurement Systems have been pioneering sorption-based solutions Carbon Capture, developing groundbreaking new instruments that enable analysis and insights never possible before.
Please download this free pack of recent scientific publications on Carbon Capture Applications of sorption science, including recent case studies and scientific overviews. If you have any questions about the topics explored, please do stop by our stand at AiCHE 2024.
Expanding the Senior Leadership Team:
Driving the Future of
Surface Measurement Systems
Surface Measurement Systems Holding LLC and its subsidiaries are entering an exciting new chapter, marked by growth, innovation, and an ambitious vision for the future. To support this next phase, we have expanded our Senior Leadership Team, ensuring we have the expertise and leadership needed to meet our goals.
This expansion includes elevating talented team members and welcoming new leaders, bringing fresh perspectives and expertise. With this enhanced team in place, SMS is better equipped than ever to drive innovation, seize emerging business opportunities, and continue improving our solutions to meet the evolving needs of our customers.
The future of Surface Measurement Systems is bright, and this leadership expansion reflects our commitment to staying at the forefront of sorption innovation, delivering exceptional value and results.
Senior Leadership Team

Europe & US Symposiums bring leading sorption scientist together
2024 saw a grand return for the live editions of the Sorption Symposium Series. With the 202 Pandemic scuppering plans for a live scientific event in 2020, Surface Measurement Systems were thrilled to be able to bring the event back to the face-to-face format, enabling a shared learning and networking experience for sorption scientists from around the world.
Eager to offer an in-person return to our users and peers around the world, the Sorption Symposium saw two live events held this year. The first, taking place in May, was held at the New Jersey Institute of Technology, while the second returned to the venue we had to cancel in 2020, University of Vienna, for our European symposium in September.

Sorption Symposium North America 2024 (7 – 9 May)
Gathers Leading Sorption Experts in New Jersey
Gathers Leading Sorption Experts in New Jersey
| The Sorption Symposium North America 2024, organized by Surface Measurement Systems, recently concluded with great success, showcasing the latest advancements in sorption science. Held in Allentown, PA, the event attracted over 60 attendees from various industries, including pharmaceuticals, environmental sciences, and material engineering. It offered a rich program featuring renowned speakers such as Dr. Maria Krisch from FreeThink Technologies and Dr. Neeraj Borker from 3M. Their presentations, alongside other experts, covered critical topics in sorption behavior, moisture analysis, and its applications across diverse fields.
A highlight of the event was the strong participation in the hands-on training sessions, where attendees received practical insights and guidance from an expert panel. The training provided an interactive platform for participants to deepen their understanding of dynamic vapor sorption (DVS) technology and its role in enhancing material performance, stability, and formulation development. This engaging format fostered an environment of collaboration and learning, elevating the educational value of the symposium. The symposium also created ample opportunities for networking, particularly during the on-site Networking Party. Attendees had the chance to connect with peers, exchange ideas, and explore potential collaborations, further strengthening the event’s impact. Overall, the Sorption Symposium North America 2024 was a resounding success, bringing together a diverse group of professionals to share knowledge, engage in hands-on experiences, and build relationships within the sorption science community. |
|
 |
 |
 |
 |
University of Vienna Plays Host to
Sorption Symposium Europe 2024 (18 – 20th Sept)
Sorption Symposium Europe 2024 (18 – 20th Sept)
|
The Sorption Symposium Europe 2024, hosted by Surface Measurement Systems, was a resounding success, gathering over 70 attendees at the University of Vienna from September 18-20. This premier event brought together leading minds from academia and industry to explore cutting-edge advancements in sorption science, particularly focusing on Dynamic Vapor Sorption (DVS) and Inverse Gas Chromatography (iGC), and recent developments in Carbon Capture Sorption Analysis capabilities. The symposium featured a stellar lineup of speakers, including Dr. Daniel Burnett and Dr. Anett Kondor from Surface Measurement Systems, along with global experts like Dr. Neeraj Borker (3M), Prof. Peter Budd (UManchester), Dr. Sam Zelinka (U.S. Forest Service), and many more. These presentations covered a broad spectrum of applications in pharmaceuticals, carbon capture, and material science, offering a comprehensive look at current research and future potential. Day three saw strong participation in hands-on workshops, where attendees received in-depth training on advanced sorption instruments, gaining valuable skills from global experts. In addition to the academic content, attendees enjoyed vibrant networking events, which included a wine and beer tasting, a traditional Austrian meal, and a unique ‘Hidden Vienna’ tour. These activities allowed participants to form lasting professional relationships in a relaxed atmosphere, enhancing the collaborative spirit of the symposium. |
|
 |
 |
 |
 |

2024: New starters
for the SMS Team
The team at Surface Measurement Systems continues to expand, with every office seeing new members join us for the exciting times ahead!
Below is a quick review of who has joined us in the last year.